International Myeloma Society Workshop on Assessment of Response and Progression Criteria in Multiple Myeloma 2024
50 $
Delivery time: Maximum to 1 hours
Format : 9 videos
File size: 4.04 GB
According to Leanne Richardson’s LinkedIn post, the IMS IMWG Multiple Myeloma Response Criteria Workshop took place in Paris in November 2024. The event focused on discussing and establishing new consensus criteria for clinical trials in multiple myeloma.
Although publicly available sources do not provide specific details about this workshop, previous workshops of the International Multiple Myeloma Working Group (IMWG) have made important contributions to standardizing response and progression criteria in the treatment of multiple myeloma. For example, international consensus response criteria for multiple myeloma were published at previous workshops, aiming to improve reporting and comparison of results between different clinical trials.
Nature
For the most up-to-date and detailed information about the conference in Paris in November 2024, you should visit the official website of the International Multiple Myeloma Society (IMS) or contact them directly.
-
Hematologists & Oncologists – Specialists involved in diagnosing and treating multiple myeloma.
-
Clinical Researchers & Scientists – Those working on new treatment strategies and response criteria for multiple myeloma.
-
Pharmaceutical & Biotech Industry Representatives – Companies developing new therapies and clinical trials.
-
Regulatory Experts & Policymakers – Authorities involved in approving and standardizing response criteria in clinical trials.
-
Medical Professionals & Fellows – Doctors and researchers looking to update their knowledge of response assessment in myeloma.
+ Topics:
Consensus Session – Response Criteria 2024.mp4
Session 1 – Monoclonal protein assessments – Response Criteria 2024.mp4
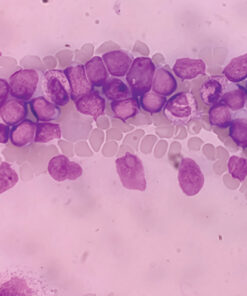
Session 2 – Bone marrow testing – Response Criteria 2024.mp4
Session 3 – Peripheral blood – Response Criteria 2024.mp4
Session 4 – Imaging for response assessment – Response Criteria 2024.mp4
Session 5 – Response assessmet in smoldering – Response Criteria 2024.mp4
Session 6 – Special situations – Response Criteria 2024.mp4
Session 7 – Defining Progression – Response Criteria 2024.mp4
Session 8 – Endpoints for clinical trial – Response Criteria 2024.mp4


 Barcelona Breast Meeting 2024
Barcelona Breast Meeting 2024  Oakstone The Brigham and Dana-Farber Board Review and Comprehensive Update in Hematology and Oncology 2025
Oakstone The Brigham and Dana-Farber Board Review and Comprehensive Update in Hematology and Oncology 2025